Drug Resistant mCRPC:
- Introduction. Accumulating evidence suggests that galectin-3 is involved in cancer progression and metastasis of many solid tumors not only by mediating neo-angiogenesis and cancer cell homotypic/heterotypic adhesions, but also by suppressing host immunity (see Figure 1).
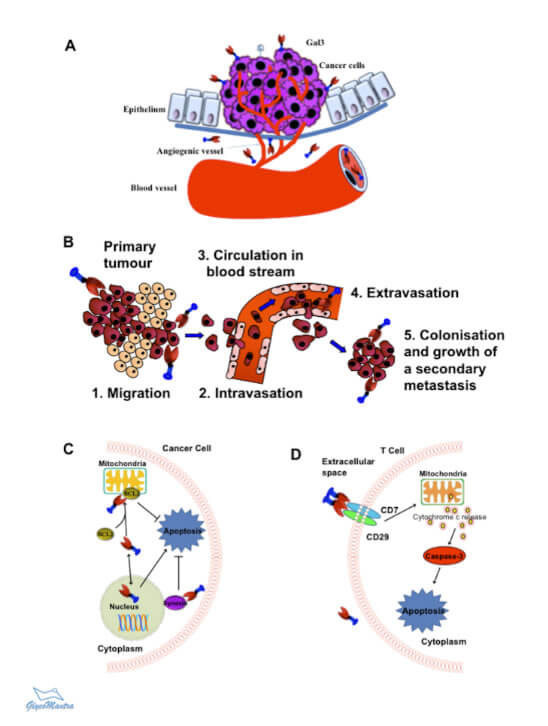 Figure 1. Key Roles of Galectin-3 in Cancer Progression. A. Tumor angiogenesis; B. Cancer metastasis; C. Cancer cell survival; and D. T-cell apoptosis.
Figure 1. Key Roles of Galectin-3 in Cancer Progression. A. Tumor angiogenesis; B. Cancer metastasis; C. Cancer cell survival; and D. T-cell apoptosis.
In case of prostate cancer, it is the leading cause of cancer-related death (375,000 death and 1.4 million new cases worldwide).
The androgen-signaling axis (hypothalamic-pituitary-gonadal axis) plays a pivotal role in the normal growth of prostate as well as the pathogenesis of prostate cancer (see Figure 2). About 80-90% of androgens (testosterone) are produced at testis. Adrenal glands produce 10-20% of androgens.
As most prostate cancers rely on the androgen receptor (AR) signaling for growth and survival, androgen deprivation therapy (ADT) has been a preferred method of treating advanced prostate cancer.
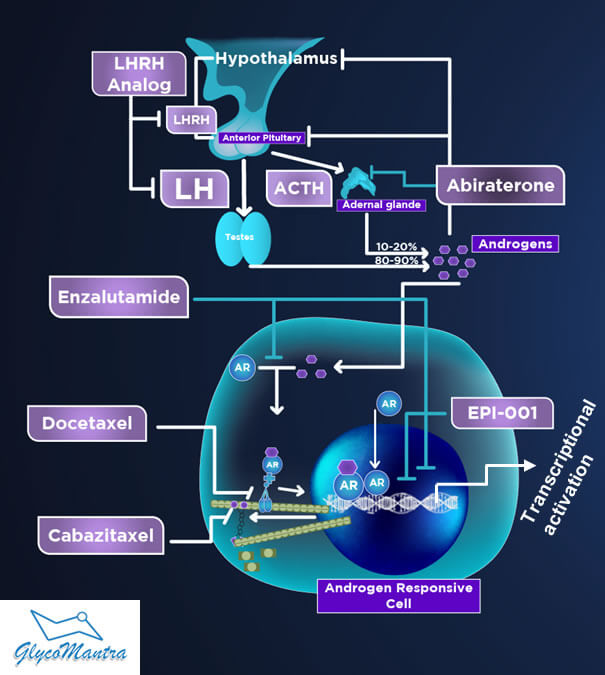
Figure 2. Androgen Receptor Signaling and Current Therapies to Treat Prostate Cancer.
How does androgen signaling work? Testosterone released from the testicles and adrenal glands is converted to the dehydrotestosterone (DHT) at the cytoplasm and binds to the AR. The activated AR-DHT complex then translocates to the nucleus and promotes AR-target genes that fuel prostate cancer growth and survival.
First-line of ADT. As a first line of therapy, the ADT is generally in the form of suppression of gonadally produced androgens via medical or surgical castration (see Figure 2).
Second-line of ADT. Unfortunately, the tumor soon grows (becomes castration resistant) and travels to distant organs (a phenomenon called “metastasis”). Although the major source of testosterone was cut off (castrated), the testosterone released from the adrenal glands may fuel the tumor growth. The selective inhibitor of androgen biosynthesis, Abiraterone (Abi), and the AR antagonist Enzalutamide (Enza) are thus recommended as a second line of homonal therapy to treat mCRPC failing the first line of ADT (See Figure 2 & 3A). The combined sale of these two drugs is about $6.5 Billion, but they extend the life of the patients by only 4.8 months.
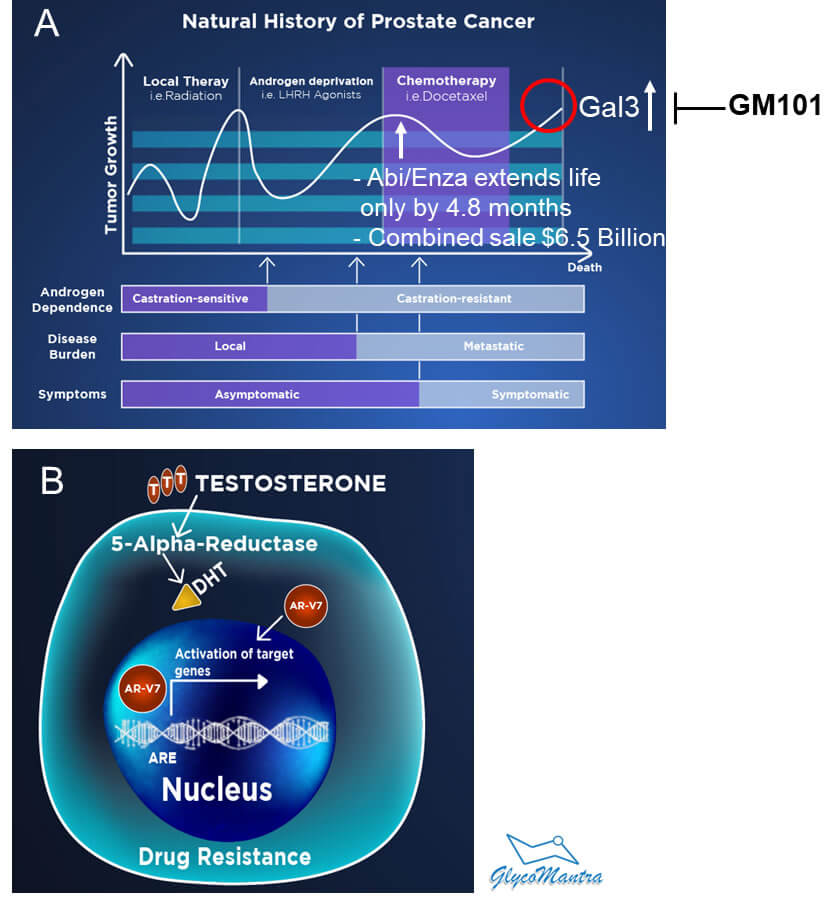 Figure 3. A. Prostate Cancer Treatment and Response. B. Role of AR-V7 in the Drug resistance of mCRPC.
Figure 3. A. Prostate Cancer Treatment and Response. B. Role of AR-V7 in the Drug resistance of mCRPC.
Our drug GM101 overcomes drug-resistance in mCRPC (unmet medical need). Abi and Enza drugs provide treatment response for a limited duration due to the development of drug resistance leading to death. Among the mechanisms leading to resistance to Abi or Enza is the expression of the AR variant, particularly AR-V7 (see Figure 3B).
Currently, no FDA-approved therapy is available for Abi/Enza-resistant mCRPC, and thus an alternative therapeutic strategy is urgently needed for this unmet medical need.
Galectin-3 is believed to cause drug resistance in mCRPC. Our GM101 drug targets galectin-3 and demonstrates pre-clinical efficacy in relevant animal model. It suppresses Abi-resistant mCRPC and increases animal survival through modulation of AR signaling (reduces expression of AR, AR-V7, phospho-Ser81-AR, and AR target genes).
The GM101 either as a stand-alone product or in combination with Abi/Enza, will be a significant arsenal against mCRPC and result in recurrence-free survival of mCRPC patients.
Relevant Publications:
-
- Liu FT, Rabinovich GA: Galectins as modulators of tumor progression. Nat Rev Cancer 2005, 5: 29-41. [PubMed]
- Johnson KD, Glinskii OV et al: Galectin-3 as a potential therapeutic target in tumors arising from malignant endothelia. Neoplasia 2007, 9: 662-670. [PubMed]
- Guha P, Bandyopadhyaya G et al: Nicotine promotes apoptosis resistance of breast cancer cells and enrichment of side population cells with cancer stem cell like properties via a signaling cascade involving galectin-3, α9 nicotinic acetylcholine receptor and STAT3. Breast Cancer Res Treat 2014, 145: 5-22. [PubMed]
- Glinskii OV, Sud S et al: Inhibition of prostate cancer bone metastasis by synthetic TF antigen mimic/galectin-3 inhibitor lactulose-l-leucine. Neoplasia 2012, 14: 65-73. [PubMed]
- Guha P, Kaptan E et al: Cod glycopeptides with picomolar affinity to galectin-3 suppresses T-cell apoptosis and prostate cancer metastasis. Proc Natl Acad Sci USA 2013, 110: 5052-5057. [PubMed]
- Dondoo T, Fukumori T et al: Galectin-3 is implicated in tumor progression and resistance to anti-androgen drug through regulation of androgen receptor signaling in prostate cancer. Anticancer Res. 2017, 37:125-134. [PubMed] The link for PubMed: 21873/anticanres.11297
- Ahmed R, Anam K, Ahmed H: Development of galectin-3 targeting drugs for therapeutic applications in various diseases. Int J Mol Sci 2023. 24:8116-8139
PubMed link:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10179732/
